Cytochrome P450 4F12 Enzyme Assay Using a Novel Bioluminescent Probe Substrate
Promega Corporation
Abstract
This is part of a series of seven articles describing new bioluminescent substrates to be used for drug screening and assaying enzymatic activity.
Introduction
CYP4F12 is a cytochrome P450 enzyme (CYP) that catalyzes the oxidation of leukotrienes, arachidonic acid and certain drugs (1) , (2) , (3) , (4) , (5) . CYP4F12 is expressed predominately in the liver with lower levels in the kidney, colon, small intestine and heart (6) . Enzyme assays for CYP4F12 typically include a chromatographic separation step (e.g., radiometric, HPLC or LC/MS-based assays), limiting the ease-of-use and throughput. Therefore, a simple, multiwell plate-based CYP4F12 enzyme assay is needed, and the luminogenic assay described here meets these criteria.
Luminogenic CYP assays use prosubstrates for the light-generating reaction of firefly luciferase. CYPs convert the prosubstrates to luciferin, which makes light in a second reaction with luciferase (7) . The amount of light generated is proportional to the amount of luciferin produced by the CYP and therefore to CYP enzyme activity (8) . Multiple CYP enzymes are encoded by families of genes in humans and other organisms (9) . The CYP enzyme selectivity for a given luminogenic substrate depends on the nature of the derivatization on the luciferin structure.
Here we demonstrated that 2-(6-(4-chlorobenzyloxy)benzo[d]thiazol-2-yl)-4,5-dihydrothiazole-4-carboxylic acid, a luciferin derivative referred to as Luciferin-4F12, was selectively converted to luciferin by the human CYP4F12 enzyme. We describe how this luciferase prosubstrate is used in a luminogenic CYP4F12 biochemical assay to detect CYP4F12 activity and CYP4F12 inhibitors, and can be used in a multiwell-plate format.
Materials and Methods
The CYP4F12 enzyme assay was performed using instructions in the P450-Glo™ Assay Technical Bulletin #TB325 and P450-Glo™ Screening Systems Technical Bulletin #TB340. The CYP enzymes used were recombinant human forms in microsomes from insect cells that coexpressed a human CYP cDNA with P450 reductase or P450 reductase plus cytochrome b5 (Gentest™ Supersomes™, BD Biosciences). A 25mM stock solution of Luciferin-4F12 (MW = 404.89) was prepared in dimethylsulfoxide (DMSO).* The CYP4F12 enzyme assays were performed using 10µM Luciferin-4F12 substrate or as indicated in figure legends, 100mM KPO4 (pH 7.4), 2nM CYP4F12 enzyme coexpressed with P450 reductase and cytochrome b5 (0.1pmol/50µl reaction), and 1X NADPH Regeneration System (Cat.# V9510).
Assays were assembled and performed in opaque white 96-well plates (e.g., white polystyrene, 96-well plates [Costar Cat.# 3912]). After incubating reactions for 10 minutes at 37°C or room temperature (20–23°C), 50µl of Luciferin Detection Reagent (LDR; Cat.# V8920, V8921) was added to each 50µl CYP reaction to stop the reactions and initiate luminescence. The luminescence was read after 20 minutes using the GloMax® 96 Microplate Luminometer (Cat.# E6501) and reported in relative light units (RLU).
For convenience, you can prepare a 4X concentrated enzyme/buffer/substrate mix (400mM KPO4 [pH 7.4], 40µM Luciferin-4F12, 8nM CYP4F12)*, 4X concentrated test compound solution (e.g., for CYP inhibition assays) and 2X concentrated NADPH Regeneration System. For a 50µl reaction, combine 12.5µl of the 4X enzyme mixture with 12.5µl of 4X test compound and initiate the reaction by adding 25µl of the NADPH Regeneration System (10) , (11)
*Note: Luciferin-4F12 solubility in KPO4 buffer is improved when the 25mM DMSO stock solution is first diluted to an intermediate concentration of 0.2mM in 100mM Tris-HCl (pH 7.5).
Results and Discussion
Substrate Selectivity
The putative luminogenic CYP substrate, Luciferin-4F12, was initially screened for activity in the luminescent assay format against 21 recombinant human CYP enzymes (Figure 1). Under the conditions used in Figure 1, we observed prominent activity with CYP4F12 and a relatively minor reactivity with CYP3A7.
 Figure 1. CYP enzyme selectivity for the Luciferin-4F12 substrate.
Figure 1. CYP enzyme selectivity for the Luciferin-4F12 substrate.
The anticipated reaction scheme with Luciferin-4F12 (I) is shown at the top. Luminescence generated by LDR depends on the conversion of I to luciferin (II). For control samples, CYP enzyme was replaced with microsomes devoid of CYP activity from the insect cell CYP expression system. Fifty microliter reactions with 50µM Luciferin-4F12 and 20nM recombinant human CYP enzymes in 96-well plates were incubated for 30 minutes at 37°C. Values are mean ± SD, n = 3.
Time Dependence
A linear time-dependent increase in luminescence reflecting luciferin accumulation was observed at room temperature (21°C) and 37°C for up to 60 minutes (Figure 2).
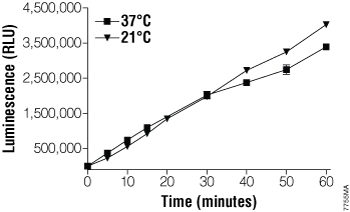 Figure 2. Time course of the CYP4F12 reaction with Luciferin-4F12.
Figure 2. Time course of the CYP4F12 reaction with Luciferin-4F12.
Time-dependent changes in net luminescence were monitored at room temperature (21°C) and 37°C. Fifty microliter reactions were performed in 96-well plates with 10µM Luciferin-4F12 and 2nM CYP4F12 in 100mM KPO4 (pH 7.4), 15mM Tris-HCl (pH 7.4). CYP reactions were initiated by staggered addition of the NADPH Regeneration System. All CYP reactions were simultaneously terminated and luciferase reactions initiated by adding 50µl of LDR. Zero-time values were measured in samples where the NADPH Regeneration System was withheld until after LDR addition. Background luminescence from control samples with no CYP enzyme (mean background = 1711 RLU at 21°C or 1636 RLU at 37°C) was subtracted to give the net luminescence values shown (mean ± SD, n = 3).
Substrate Concentration
CYP4F12 activity increased in a dose-dependent manner up to a maximum at 25µM Luciferin-4F12 (Figure 3). A decrease in activity was observed at higher concentrations (data not shown); however, the data did not conform well to a substrate inhibition model (10) . Since a nonspecific effect (e.g., substrate insolubility) at high concentrations could not be ruled out, the data up to 25µM was fit to a standard hyperbolic function that estimates an apparent Km of 10µM.
 Figure 3. Km measurement of CYP4F12 using Luciferin-4F12.
Figure 3. Km measurement of CYP4F12 using Luciferin-4F12.
Reactions with 2nM CYP4F12 and 100mM KPO4 (pH 7.4) were incubated for 10 minutes at 37°C. The curve fit and Km calculation were done using GraphPad Prism® software. Background luminescence from control samples with no CYP enzyme at each substrate concentration was subtracted to give the net luminescence values shown (mean ± SD, n = 3).
CYP4F12 Inhibition
The CYP4F12 assay with 2nM CYP4F12 was tested as a tool for measuring CYP4F12 inhibition with 10µM Luciferin-4F12, the apparent Km concentration (Figure 4). We observed dose-dependent inhibition by astemizole, a CYP4F12 substrate acting as a competitive inhibitor of the CYP4F12 reaction with Luciferin-4F12 (3) .
 Figure 4. Measuring CYP4F12 inhibition using Luciferin-4F12.
Figure 4. Measuring CYP4F12 inhibition using Luciferin-4F12.
Using 2nM CYP4F12, enzyme inhibition was assayed using 10µM Luciferin-4F12 in the presence of astemizole at the indicated concentrations. The astemizole was diluted from a 20mM stock solution in DMSO, and the amount of this vehicle was kept constant at 0.1% in all reactions. Reactions were incubated at 37°C for 10 minutes before adding LDR. Background luminescence from control samples with no CYP enzyme (mean background = 693 RLU) was subtracted to give the net luminescence values shown (mean ± SD, n = 3).
Conclusion
Luciferin-4F12, a novel luciferin derivative, is a luminogenic cytochrome P450 substrate with a high degree of selectivity for CYP4F12. Using Luciferin-4F12 with the luminogenic CYP enzyme assay approach harnesses the exquisite sensitivity, selectivity and simplicity of bioluminescence. This provides for simple, rapid, multiwell plate-based CYP4F12 enzyme assays.
Related Products
Article References
- Hashizume, T. et al. (2001) Cytochrome P450 4F12 enzyme assay using a novel bioluminescent probe substrate. Bioch. Biophys. Res. Com. 280, 1135–41.
- Stark, K. et al. (2004) Expression of CYP4F12 in the gastrointestinal and urogenital epithelia. Basic Clin. Phar. Tox. 94, 177–83.
- Parikh, S. et al. (2003) CYP2J2 and CYP4F12 are active for the metabolism of non-sedating antihistamines: terfenadine and astemizole. Drug. Met. Rev. 35, 190–1.
- Matsumoto, S. et al. (2002) Involvement of CYP2J2 on the intestinal first-pass metabolism of antihistamine drug, astemizole. Drug Metab. Dispos. 30, 1240–5.
- Hashizume, T. et al. (2002) Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in human intestinal microsomes. J. Pharm. Exp. Therap. 300, 298–304.
- Bylund, J., Bylund, M. and Oliw, E.H. (2001) cDNA cloning and expression of CYP4F12, a novel human cytochrome P450. Bioch. Biophys. Res. Com. 280, 892–7.
- Branchini, B.R. et al. (1998) Site-directed mutagenesis of histidine 245 in firefly luciferase: A proposed model of the active site. Biochemistry 37, 15311–9.
- Guengerich, F.P. (2006) Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 8, E101–11.
- Cali, J.J. et al. (2006) Luminogenic cytochrome P450 assays. Exp. Op. Drug Metab. Toxicol. 2, 629–45.
- P450-Glo™Assays Technical Bulletin, TB325, Promega Corporation.
- P450-Glo™ Screening Systems Technical Bulletin, TB340, Promega Corporation.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Sobol, M., Ma, D., Woodroofe, C. C. and Cali, J. J. Cytochrome P450 4F12 Enzyme Assay Using a Novel Bioluminescent Probe Substrate. [Internet] . [cited: year, month, date]. Available from: https://www.promega.com/resources/pubhub/enotes/cytochrome-p450-4f12-enzyme-assay-using-a-novel-bioluminescent-probe-substrate/
American Medical Association, Manual of Style, 10th edition, 2007
Sobol, M., Ma, D., Woodroofe, C. C. and Cali, J. J. Cytochrome P450 4F12 Enzyme Assay Using a Novel Bioluminescent Probe Substrate. Promega Corporation Web site. https://www.promega.com/resources/pubhub/enotes/cytochrome-p450-4f12-enzyme-assay-using-a-novel-bioluminescent-probe-substrate/ Accessed Month Day, Year.
Products may be covered by pending or issued patents or may have certain limitations on use. Please visit our patent and trademark web page for more information.
GloMax is a registered trademark of and P450-Glo is a trademark of Promega Corporation.
Gentest and Supersomes are trademarks of Becton, Dickinson and Company. GraphPad Prism is a registered trademark of GraphPad Software, Inc.
 Learn about the chemistry of the bioluminescent reaction and its use in a broad range of assays.
Learn about the chemistry of the bioluminescent reaction and its use in a broad range of assays.