Nonselective Cytochrome P450 Enzyme Assay Using a Novel Bioluminescent Probe Substrate that Cross-Reacts with Multiple P450 Enzymes
Promega Corporation
Publication Date: 2008
Abstract
This is part of a series of seven articles describing new bioluminescent substrates to be used for drug screening and assaying enzymatic activity.
Introduction
The cytochromes P450 (CYPs) are a large family of enzymes that catalyze the oxidation of numerous hydrophobic chemicals, including endogenous compounds, therapeutic drugs and environmental toxins (1) . Known CYP substrates are used as probes for determining how a compound might interact with CYPs as substrate or regulator (2) . A highly selective probe substrate is desirable when targeting a single CYP in a mix of other potentially cross-reacting enzymes. In contrast, a promiscuous probe substrate is useful for applications such as characterizing multiple CYP enzymes in isolation, measuring net CYP activity in a mixture, or for mutagenesis studies that can benefit from a substrate with loose active-site-binding constraints.
Luminogenic CYP assays use prosubstrates for the light-generating reaction of firefly luciferase. CYPs convert the prosubstrates to luciferin or a luciferin ester, which produces light in a second reaction with a luciferase reaction mixture called Luciferin Detection Reagent (LDR) (3) (4) . The amount of light is proportional to the amount of luciferin produced by the CYP, and therefore, to CYP enzyme activity. Multiple CYP enzymes are encoded by families of genes in humans and other organisms (5) . The CYP enzyme selectivity for a given luminogenic substrate depends on the nature of the derivatization on the luciferin structure. Here we demonstrate that methyl 2-(6-methoxybenzo[d]thiazol-2-yl)-4,5-dihydrothiazole-4-carboxylate, a promiscuous CYP substrate referred to as Luciferin-MultiCYP, reacts with at least 21 CYP enzymes and can be used in a convenient, multiwell-luminogenic assay format.
Materials and Methods
The CYP enzyme assays were performed using instructions in the P450-Glo™ Assay Technical Bulletin #TB325 and P450-Glo™ Screening Systems Technical Bulletin #TB340. The CYP enzymes used were recombinant human forms in microsomes from insect cells that coexpressed a human CYP cDNA with P450 reductase (Gentest™ Supersomes™, BD Biosciences). The CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, CYP2J2, CYP3A4, CYP3A5, CYP3A7, CYP4F2, CYP4F3A, CYP4F3B and CYP4F12 microsomes also were coexpressed with cytochrome b5. A 50mM stock solution of Luciferin-MultiCYP (molecular weight = 308.37) was prepared in acetonitrile.
CYP 2X concentrated enzyme/buffer/substrate mixes were prepared with 40nM CYP enzyme, 100µM Luciferin-MultiCYP substrate and 2X buffer concentrations recommended by the CYP enzyme manufacturer (1X buffer composition: 100mM KPO4 (pH 7.4) for CYP1A1, CYP1A2, CYP1B1, CYP2B6, CYP2D6, CYP2E1, CYP2J2, CYP3A5, CYP3A7, CYP4F12, CYP19 and Control; 50mM KPO4 (pH 7.4) for CYP2C8, CYP2C19, CYP4F2, CYP4F3A, CYP4F3B; 25mM KPO4 (pH 7.4) for CYP2C9; 200mM KPO4 (pH 7.4) for CYP3A4; 100mM Tris HCl (pH 7.5) for CYP2A6, CYP2C18 and CYP4A11). Twenty five microliters of each 2X enzyme/buffer/substrate mix was added to opaque white 96-well plates (e.g., white polystyrene, 96-well plates [Costar Cat.# 3912]). Reactions were initiated by adding 25µl of 2X NADPH Regeneration System (Cat.# V9510) and incubated for 30 minutes at 37°C. To stop the reactions, 50µl of Luciferin Detection Reagent (LDR) with esterase (Cat.# V8930, V8931) was added to each well. Luminescence was read after 20 minutes with the GloMax® 96 Microplate Luminometer and reported in relative light units (RLU).
Results and Discussion
The putative luminogenic CYP substrate Luciferin-MultiCYP was screened for activity in the P450-Glo™ luminescent assay format against 21 recombinant human CYP enzymes (Figure 1) (6) (7) . Under the conditions used in Figure 1, each of the 21 enzymes exhibited activity with the Luciferin-MultiCYP substrate.
 Figure 1. Activity of CYP enzymes with Luciferin-MultiCYP substrate.
Figure 1. Activity of CYP enzymes with Luciferin-MultiCYP substrate.
The anticipated reaction scheme with Luciferin-MultiCYP (I) is shown at the top. Luminescence generated by LDR depends on the conversion of I to luciferin methyl ester (II). For control samples, CYP enzyme was replaced with microsomes devoid of CYP activity from the insect cell CYP expression system. Fifty microliter reactions with 50µM Luciferin-MultiCYP and 20nM recombinant human CYP enzymes were performed in 96-well plates and incubated for 30 minutes at 37°C. Values are mean ± SD, n = 3.
The CYP activities can be arbitrarily ranked as high, medium and low based on the relative magnitude of the signal:background ratios (S:B; Table 1). The high S:B group includes CYP1A1, CYP1A2, CYP1B1 and CYP2D6, with CYP1A2 exhibiting the strongest activity. The medium S:B group includes CYP2B6, CYP2C9, CYP2C19, CYP2J2, CYP3A4, CYP3A5, CYP4A11, CYP4F2, CYP4F3A, CYP4F3B, CYP4F12 and CYP19. The low S:B group includes CYP2A6, CYP2C8, CYP2C18, CYP2E1 and CYP3A7, which showed the lowest activity. Although the S:B for all the CYP reactions with Luciferin-MultiCYP are sufficient assay starting points, additional characterization of each reaction will be necessary. For applications such as inhibitor screening or CYP enzyme characterizations, initial analysis of reaction time, temperature, substrate-dose dependence and enzyme concentration should be performed.
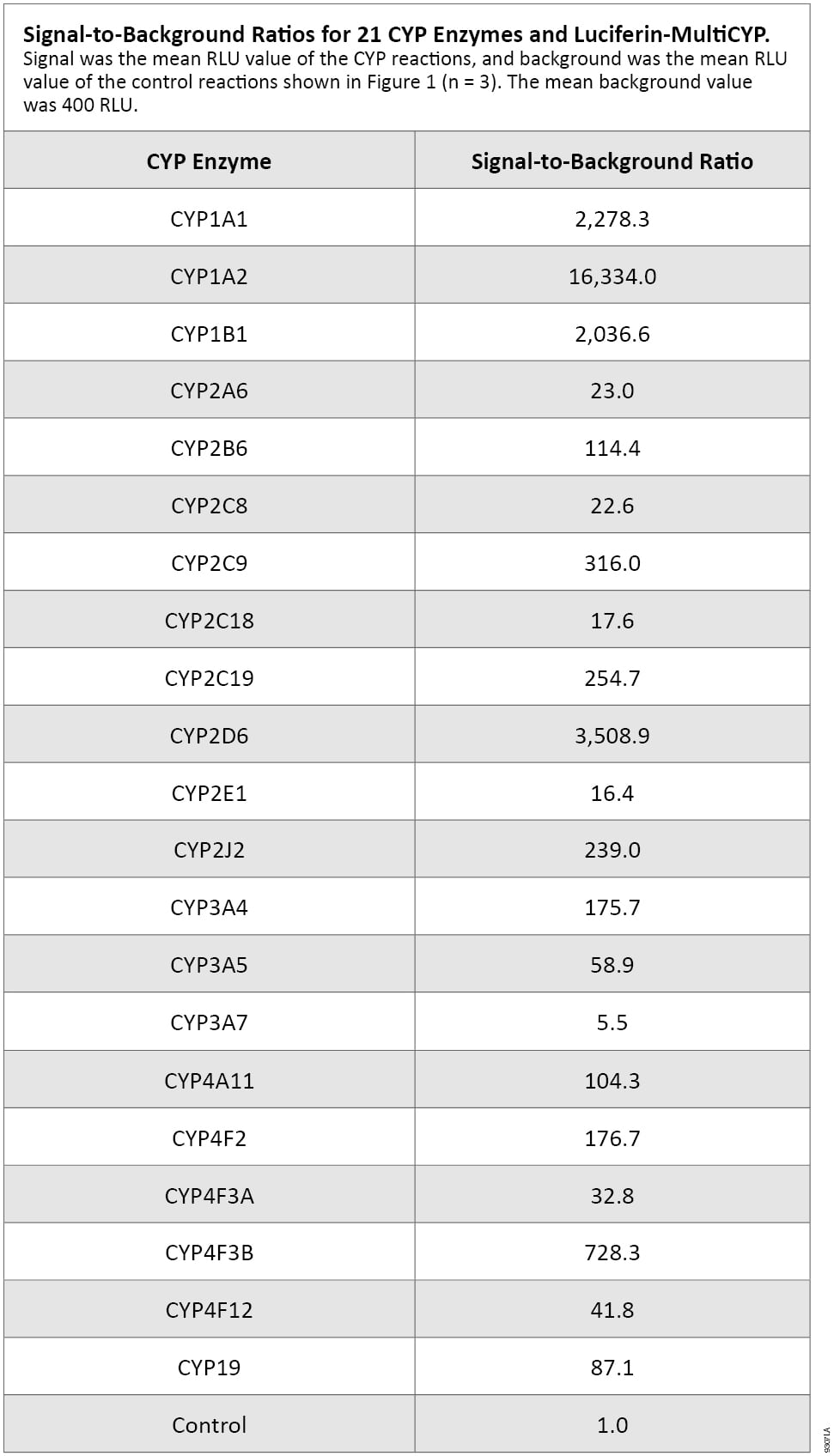 Table 1. Signal-to-Background Ratios for 21 CYP Enzymes and Luciferin-MultiCYP.
Table 1. Signal-to-Background Ratios for 21 CYP Enzymes and Luciferin-MultiCYP.
Signal was the mean RLU value of the CYP reactions, and background was the mean RLU value of the control reactions shown in Figure 1 (n = 3). The mean background value was 400 RLU.
Conclusion
Luciferin-MultiCYP, a novel luciferin derivative, is a promiscuous CYP substrate that can be used to assay numerous CYP enzymes. Using Luciferin-MultiCYP for CYP enzyme assays harnesses the exquisite sensitivity and simplicity of bioluminescence. This provides for simple, rapid, multiwell plate-based CYP enzyme assays.
Related Products
Related Resources
Article References
- Wrighton, S.A. and Stevens, J.C. (1992) The human hepatic cytochromes P450 involved in drug metabolism. Crit. Rev. Tox. 22, 1–21.
- Bjornsson, T.D. et al. (2003) The conduct of in vitro and in vivo drug-drug interaction studies: A Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab. Dispo. 31, 815–32.
- Branchini, B.R. et al. (1998) Site-directed mutagenesis of histidine 245 in firefly luciferase: A proposed model of the active site. Biochemistry 37, 15311–9.
- Cali, J.J. et al. (2006) Luminogenic cytochrome P450 assays. Exp. Op. Drug Metab. Toxicol. 2, 629–45.
- Guengerich, F.P. (2006) Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 8, E101–11.
- P450-Glo™Assays Technical Bulletin, TB325, Promega Corporation.
- P450-Glo™ Screening Systems Technical Bulletin, TB340, Promega Corporation.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Sobol, M., Ma, D., Unch, J. and Cali, J. J. Nonselective Cytochrome P450 Enzyme Assay Using a Novel Bioluminescent Probe Substrate that Cross-Reacts with Multiple P450 Enzymes. [Internet] 2008. [cited: year, month, date]. Available from: https://www.promega.com/resources/pubhub/enotes/nonselective-cyp450-assay-using-a-biolum-probe-substrate-that-cross-reacts-with-multiple-p450s/
American Medical Association, Manual of Style, 10th edition, 2007
Sobol, M., Ma, D., Unch, J. and Cali, J. J. Nonselective Cytochrome P450 Enzyme Assay Using a Novel Bioluminescent Probe Substrate that Cross-Reacts with Multiple P450 Enzymes. Promega Corporation Web site. https://www.promega.com/resources/pubhub/enotes/nonselective-cyp450-assay-using-a-biolum-probe-substrate-that-cross-reacts-with-multiple-p450s/ Updated 2008. Accessed Month Day, Year.
Products may be covered by pending or issued patents or may have certain limitations on use. Please visit our patent and trademark web page for more information.
GloMax is a registered trademark of and P450-Glo is a trademark of Promega Corporation.
Gentest and Supersomes are trademarks of Becton, Dickinson and Company.
 Learn about the chemistry of the bioluminescent reaction and its use in a broad range of assays.
Learn about the chemistry of the bioluminescent reaction and its use in a broad range of assays.